What Does PQRI Do?
-
- Unites thought leaders from regulatory agencies, standard setting bodies, industry and academia to conduct research and share knowledge on emerging scientific and regulatory quality challenges
- Provides a unique, neutral forum to develop broad consensus among a diverse collection of industry organizations and regulatory bodies
- Creates opportunities to accomplish mutual goals that cannot be achieved by individual organizations alone, by leveraging the energy, resources, and intelligence of leading global organizations
- Impacts global regulatory guidance and standards, bringing maximum value to members and patients
What Makes PQRI Unique?
-
- PQRI’s inclusion of regulatory agencies and standard-setting bodies as members as well as its distinct organizational structure, allows for direct connection between regulators, academia and industry and fosters cross-collaborative pathways between these various stakeholders
- PQRI provides resources to support research projects that serve as stimuli for and help shape global regulatory policies
- PQRI helps its member organizations meet their missions by identifying work of broad interest to those organizations’ members
- PQRI provides a platform that encourages and facilitates inter-organizational collaboration
Organizational Structure
PQRI Organizational Chart
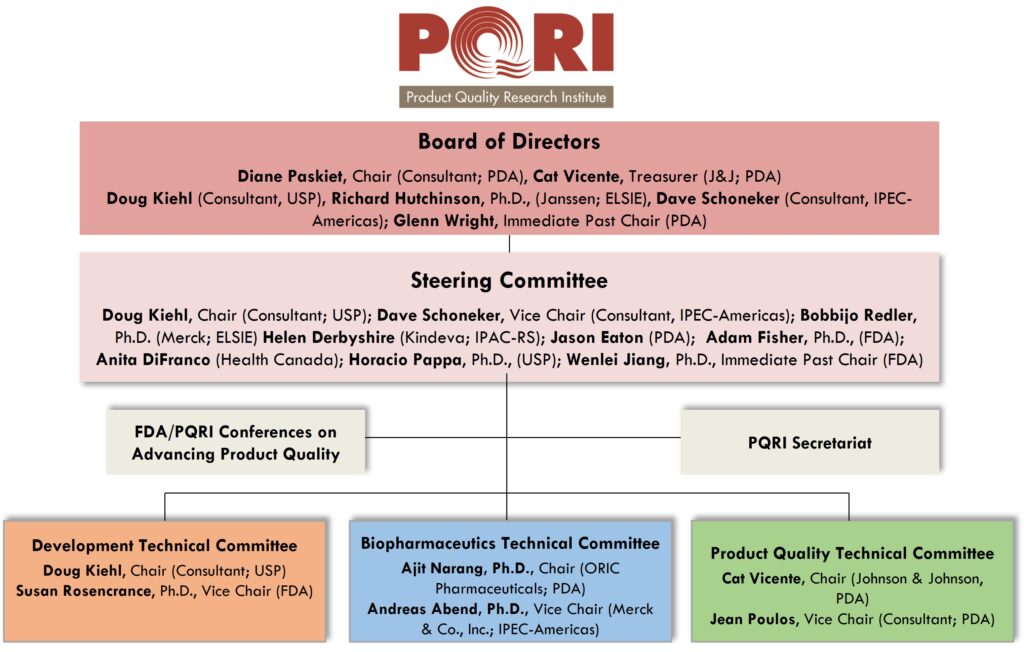
The PQRI Board of Directors and Steering Committee are the dual governing bodies of PQRI.
Board of Directors*
The Board of Directors is vested with the administrative management, growth, and operation of the Institute, except for those activities involving scientific decision making, which are delegated to the PQRI Steering Committee. The Board has authority over the collection and disbursement of funds to ensure the effective operation of the Institute.
-
-
-
-
- Each non-government member organization is entitled to nominate members to be elected to the Board, which consists of five seats, including the Chair and Treasurer.
Steering Committee*
The PQRI Steering Committee has sole authority over all scientific activities conducted under the auspices of the Institute and is responsible for recommending the disbursement of funds for those activities, to the Board of Directors.
-
-
-
-
-
- Each member organization is entitled to representation on the Steering Committee and one vote on requiring matters.
Technical Committees*
PQRI consists of three Technical Committees that provide technical and scientific guidance, direction and review for PQRI Working Groups. The Technical Committees consist of scientists and regulatory experts from industry, academia, and regulatory agencies, and make technical/scientific recommendations to the Steering Committee.
Working Groups
PQRI has several very active Working Groups operating under the guidance of each Technical Committee. These Working Groups consist of scientists from industry, academia and FDA, who generate, evaluate, and discuss information or data, and develop PQRI recommendations, technical reports, and scientific papers. See the above Technical Committee links for information on PQRI Working Groups.
* Copies of minutes are available upon request. Please contact the PQRI Secretariat for copies of minutes.


